Named reactions are one of the most important yet misunderstood topics in organic chemistry. Many students feel overwhelmed because there are too many names, reagents, and conditions. However, in reality, named reactions are among the highest-scoring areas in CBSE board exams and JEE.
Every year, board papers include direct questions, conversions, and reasoning based on named reactions. Similarly, competitive exams test how well students apply these reactions, not just remember them. Therefore, learning named reactions properly can significantly boost your chemistry score.
This blog is written to help Class 12 students understand named reactions in organic chemistry clearly. You will learn why they matter, which reactions are most important, and how to study them effectively. Most importantly, you will see how smart tools like Super Tutor can simplify learning and revision.
What Are Named Reactions in Organic Chemistry

Named reactions are organic chemical reactions named after scientists. Each reaction represents a specific chemical transformation. Instead of memorising random steps, students learn a standard reaction pattern. As a result, complex organic chemistry becomes easier to manage.
In organic named reactions, examiners usually test:
- Correct identification of reagents
- Understanding of reaction conditions
- Ability to predict final products
When students understand these elements together, accuracy improves naturally.
Why Named Reactions Are Important for CBSE Board Exams and JEE

In the name reactions class 12, CBSE repeatedly asks direct and indirect questions. These include writing reactions, completing conversions, and explaining reaction logic. Moreover, CBSE strictly follows NCERT examples and in-text reactions. Therefore, mastering NCERT-based named reactions guarantees safe marks.
From an exam perspective, named reactions matter because:
AI-powered learning platforms bring several benefits to students. Let’s look at some important ones:
- Board exams often include direct theory questions, such as defining or explaining reactions like Hoffmann Bromamide Degradation.
- In organic conversions, named reactions are essential to transform one compound into another correctly.
- Mechanism-focused questions in JEE Mains test your understanding of intermediates and step-by-step reaction pathways.
- Several named reactions also act as identification tests, helping detect specific functional groups.
- A fixed and predictable weightage is allotted to these reactions in CBSE board papers.
- Both conversion-based and reasoning questions heavily rely on a solid grip of named reactions.
- Most JEE Mains organic chemistry problems are built on the fundamentals of these reactions.
Students can verify the official syllabus and chapter weightage here on the CBSE official website.
List of Important Name Reactions in Organic Chemistry Class 12
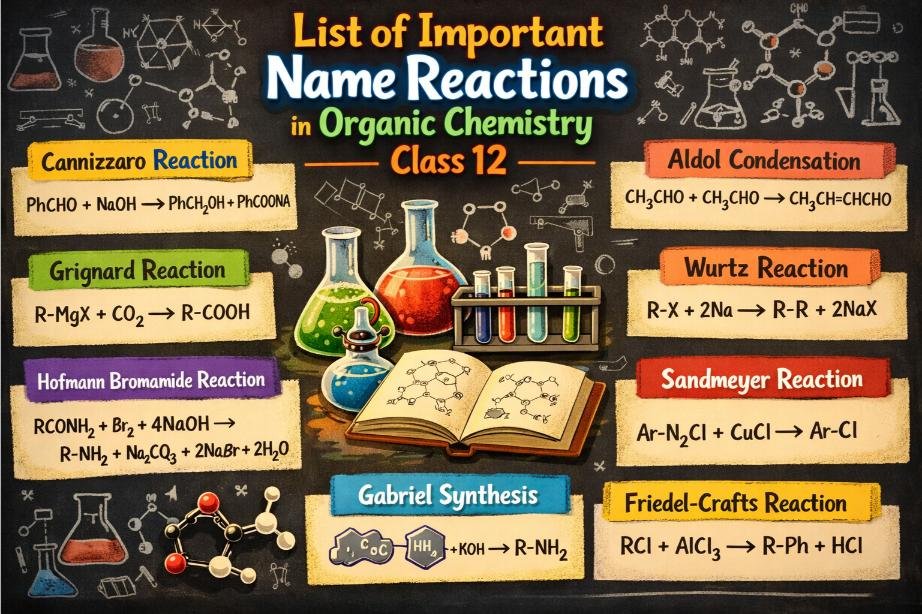
To make learning easier and more systematic, important named reactions are best studied chapter-wise. This categorisation helps students connect reactions directly with their NCERT chapters and exam questions.
Finkelstein Reaction: An SN2 halogen exchange reaction where alkyl chlorides or bromides are converted into alkyl iodides using NaI in dry acetone. The reaction proceeds forward because NaCl or NaBr precipitates out.
Swarts Reaction: Used to prepare alkyl fluorides by heating alkyl chlorides or bromides with metal fluorides such as AgF, Hg2F2, or SbF3. Direct fluorination is difficult, which makes this reaction important.
Sandmeyer Reaction: Used to convert aniline into aryl halides. Aryl diazonium salts react with copper(I) salts like CuCl, CuBr, or CuCN to form substituted aromatic compounds.
Wurtz Reaction: Two alkyl halides react with sodium metal in dry ether to form a higher alkane. This reaction is useful for increasing carbon chain length.
- Reimer–Tiemann Reaction: Phenol reacts with chloroform (CHCl3) and aqueous NaOH to introduce a formyl group (–CHO) at the ortho position, forming salicylaldehyde.
- Kolbe’s Reaction: Phenoxide ion reacts with carbon dioxide (CO2) under pressure, followed by acidification, to produce salicylic acid. This reaction demonstrates ortho-directing effects in phenols.
- Williamson Ether Synthesis: A primary alkyl halide reacts with an alkoxide ion via an SN2 mechanism to form ethers. This is the most common method for ether preparation.
Aldol Condensation: Aldehydes or ketones containing alpha-hydrogen react with dilute alkali to form beta-hydroxy carbonyl compounds. On heating, these compounds dehydrate to form alpha, beta-unsaturated compounds.
Cannizzaro Reaction: Aldehydes lacking alpha-hydrogen undergo self-oxidation and reduction in concentrated alkali. One molecule forms an alcohol, while the other forms a carboxylic acid salt.
Clemmensen Reduction: Aldehydes or ketones are reduced to alkanes using zinc amalgam (Zn/Hg) and concentrated HCl. This reduction works best under acidic conditions.
Wolff–Kishner Reduction: Carbonyl compounds are converted into alkanes using hydrazine (NH2NH2) and KOH in ethylene glycol. This method is preferred under strongly basic conditions.
Rosenmund Reduction: Acyl chlorides are selectively reduced to aldehydes using hydrogen gas and palladium on barium sulphate (Pd/BaSO4). The catalyst is poisoned to prevent over-reduction.
- Etard Reaction: Chromyl chloride (CrO2Cl2) is used to oxidise the methyl group of toluene to benzaldehyde. This reaction is specific for aromatic side-chain oxidation.
- Hell–Volhard–Zelinsky (HVZ) Reaction: Carboxylic acids undergo halogenation at the alpha position using red phosphorus (P) and halogen (Cl2 or Br2). The reaction requires the presence of alpha-hydrogen.
Gabriel Phthalimide Synthesis: A three-step reaction used to prepare pure primary aliphatic amines from primary alkyl halides. This method avoids the formation of secondary and tertiary amines.
Hoffmann Bromamide Degradation: Primary amides react with bromine (Br2) and sodium hydroxide (NaOH) to form primary amines with one less carbon atom. Carbon dioxide (CO2) is released during the reaction.
Carbylamine Reaction: A qualitative test for primary amines. Primary amines react with chloroform (CHCl3) and alcoholic KOH to form foul-smelling isocyanides.
For higher-level preparation, students must also understand all name reactions in organic chemistry JEE mains.
Fischer Indole Synthesis: A classical method for synthesising indoles by reacting phenylhydrazines with aldehydes or ketones under acidic conditions.
Walden Inversion: The complete (100%) inversion of optical configuration at a chiral centre that occurs during an SN2 reaction.
Diels–Alder Reaction: A concerted [4+2] cycloaddition between a conjugated diene and a dienophile, resulting in the formation of six-membered cyclic compounds.
Michael Addition: A nucleophilic 1,4-addition in which a carbanion or other nucleophile adds to an α,β-unsaturated carbonyl compound.
Heck Reaction: A palladium-catalysed coupling reaction that joins aryl or vinyl halides with alkenes to form substituted alkenes.
Lindlar Catalyst: A selective catalyst (Pd/CaCO₃ poisoned with lead) used for the partial reduction of alkynes to cis-alkenes.
Robinson Annulation: A two-step reaction sequence involving a Michael addition followed by intramolecular aldol condensation to construct fused six-membered ring systems.
How to Study Named Reactions Class 12 Effectively

Mastering all name reactions requires a shift from rote memorisation to active conceptual application. Simply reading a name reactions class 12 pdf is rarely enough for long-term retention. Instead, follow these proven strategies to dominate your chemistry papers:
- Create a Reagent Map: Think of reagents as the “keys” that unlock specific chemical doors. Whenever you see 𝑍𝑛(𝐻𝑔)/𝐻𝐶𝑙, your mind should instantly snap to Clemmensen Reduction. Group your organic name reactions by the reagents they use to avoid confusion during the exam.
- Visualise the Electron Flow: For all name reactions in organic chemistry JEE mains, understanding the mechanism is paramount. Do not just memorise the final product; draw the curved arrows to show how electrons move. This “muscle memory” of drawing mechanisms will help you predict products for reactions you haven’t even seen before.
- Build a Comparative Index: Many named reactions in organic chemistry pdf files look similar but have subtle differences. For instance, both Aldol and Cannizzaro involve aldehydes, but their requirements for α-hydrogen are opposite. Create a “Comparison Table” to contrast these look-alike reactions side-by-side.
- The “Daily Five” Rule: Instead of cramming all name reactions in one night, pick five reactions every morning. Write down their reactants, reagents, and products from memory. By the end of two weeks, you will have mastered the entire name reactions class 12 syllabus.
How Super Tutor Helps You Master Name Reactions

Learning all the name reactions alone can be a bit boring and difficult. Super Tutor makes the whole process much easier and more organised.
Super tutor provides everything in one place, so you don’t have to waste time searching:
- Revision Notes: You get access to clear, digital revision notes that are very easy to follow and study from.
- Flashcards: These are great for testing yourself on reagents when you have a few minutes free.
- Study Slides: Super tutor’s study slides show organic named reactions step-by-step, making them easy to see and understand.
- Syllabus Section Lists: We have a special section for the list of formulas and reagents. This helps you find exactly what you need for all name reactions quickly.
Super Tutor knows which name reactions class 12 are most likely to come in the exam. It helps students focus on the most important points to score full marks in the CBSE board exams.
Final Revision Tips for Named Reactions

As the exams get closer, the goal is not to learn new reactions but to strengthen recall and accuracy. At this stage, a well-planned revision routine can make a noticeable difference in exam performance. To make the final revision effective, follow these focused strategies:
- Map it out: Create a giant wall chart of all name reactions, or just go to Super tutor and use revision notes to get everything in one place.
- Flashcards: Revise reagents and products using flashcards rather than rereading notes. This strengthens memory and improves reaction recognition speed.
- NCERT Focus: Ensure you follow the official NCERT Chemistry Part II for board-accurate definitions.
- Solve PYQs: Look at how name reactions class 12 were tested in the last five years by solving PYQs on Super Tutor.
Conclusion
Named reactions are one of the most dependable scoring areas in organic chemistry. They appear consistently in CBSE board exams and play a crucial role in competitive exams like JEE. When studied with structure and clarity, they stop being overwhelming and start working in your favour.
The key to mastering named reactions lies in understanding patterns, reagents, and applications rather than memorising isolated facts. Organised study, regular revision, and focused practice make these reactions easy to recall under exam pressure. Using tools like revision notes, flashcards, and reaction lists further strengthens retention.
With a clear strategy and the right resources, students can turn named reactions into a strong advantage. Super Tutor supports this journey by providing structured learning, revision-friendly content, and syllabus-aligned materials. Start preparing early, revise smartly, and approach your exams with confidence.